Radioactive elements decay in one of three ways:
A nucleus can eject 2 protons+2 neutrons (an alpha particle) and transform into a less massive element [alpha decay]
A neutron can turn into a proton+electron. The electron leaves [beta decay] and the atomic number goes up by 1.
Fission -- the nucleus splits into two new (smaller) atoms.
Gamma rays (neutrally charged) can also be emitted from various radioactive
elements.
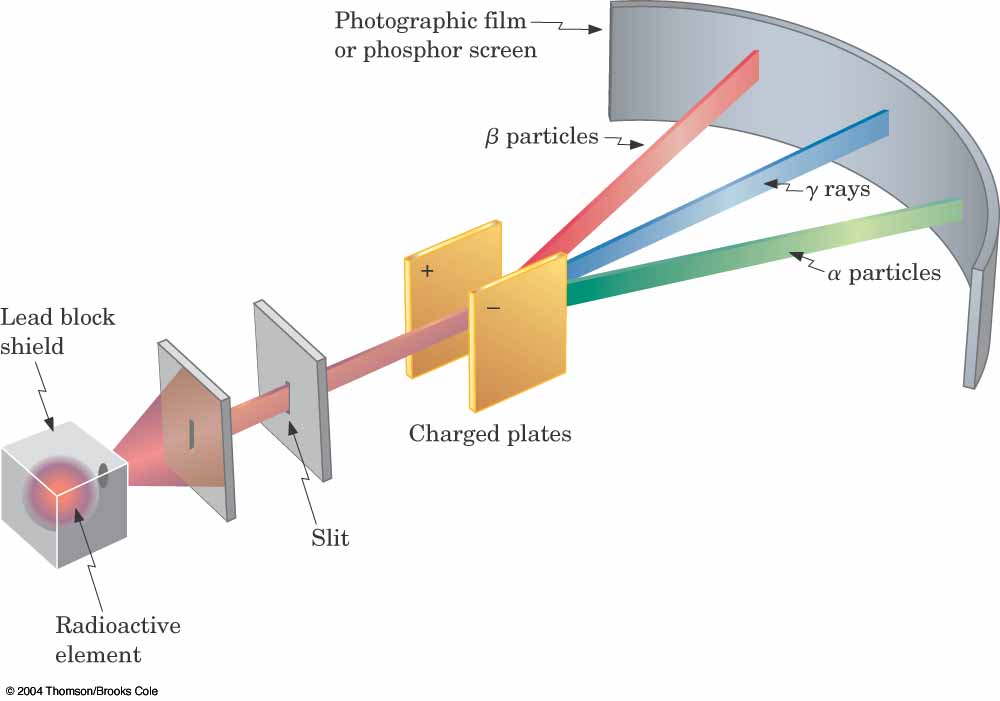
Bettelheim et al.
In class exercise: Explain what's going on in this
figure.
Back
Next
Back to Lecture 6 outline
Back to main lectures page
Back to main course page

