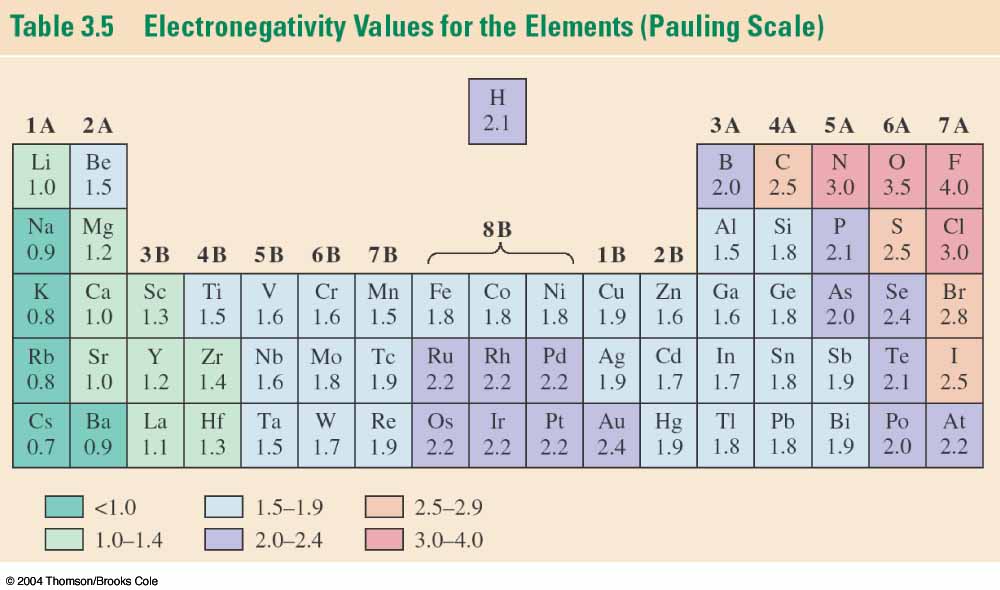
The answer lies here:

Carbon has a substantially higher
electronegativity than the other
elements in its column.
This means that carbon holds onto its
electrons tighter, which in turn means
that its bonds are stronger. Thus,
a C-C bond is stronger than
a Si-Si bond.
Remember, though, what happened when
we put three different substances in water:
the ionically bonded compound
easily fell apart in the polarized water.
So organic molecules are going to have to
deal with this problem too: they need to
have strong bonds (to stay together), but
if they are too ionic, they could dissolve
in liquid.