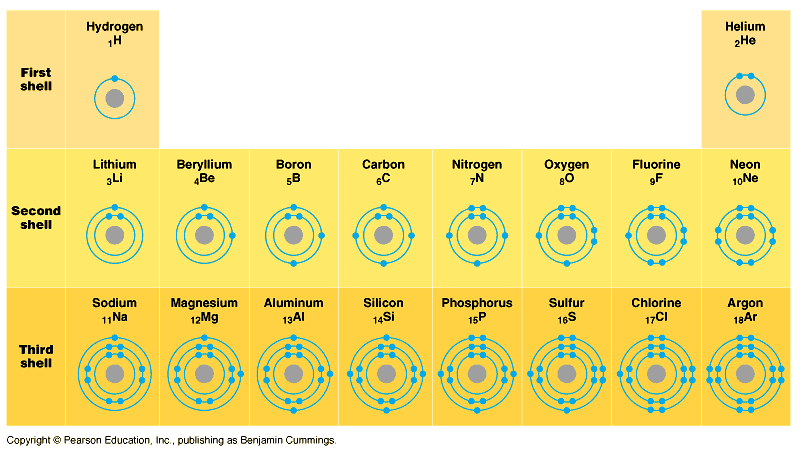
We also now know enough to talk about chemical bonds.
It turns out that atoms like to have complete energy levels,
which means either taking electrons in to fill up,
or giving electrons away to fill down.

Helium has a complete shell already: 1s holds 2 electrons.
Neon also is complete: 2 electrons in 1s, 2 electrons in
2s, and 6 electrons in 2p.
Argon has two complete orbitals but not a complete shell:
1s (2), 2s (2), 2p (6), 3s (2), 3p (6), and none in 3d.
Of course, 4s comes before 3d.